
The second one is forgetting to have both the entropy change and the enthalpy change in the same units - either kJ Or J. I was recently studying electrolysis and they talked about hooking up an electrochemical cell to a power supply and using electrical energy to drive a. Non-Spontaneous Process is a term that refers to a process that does not happen spontaneously. The first one is forgetting the temperature needs to be in Kelvin and using Centigrade directly The entropy change and be calculated from the entropy products, minus the entropies of the reactants.Īlternatively, if you know the enthalpy change and the entropy change, you an set #DeltaG = 0#, which is the point at which the reaction goes from being in not feasible to feasible, and use this to calculate #T#, the temperature at which the reaction becomes feasible. The application of spontaneity with respect to equilibrium is as follows: A reaction will never.
The enthalpy change can be calculated using a Hess cycle if you don' know it, using enthalpy changes of other reactions which are known. A non-spontaneous process needs a continual input of energy. T is the temperature of the reaction, in KelvinĪnd #DeltaS# is the entropy change (in #kJ K^(-1) mol^(-1)#) #DeltaH# is the enthalpy change (in #kJ mol^-1#) Spontaneous reactions occur without any input into the reaction, non-spontaneous reactions need input such as extra energy.
#Spontaneous and non spontaneous reaction free#
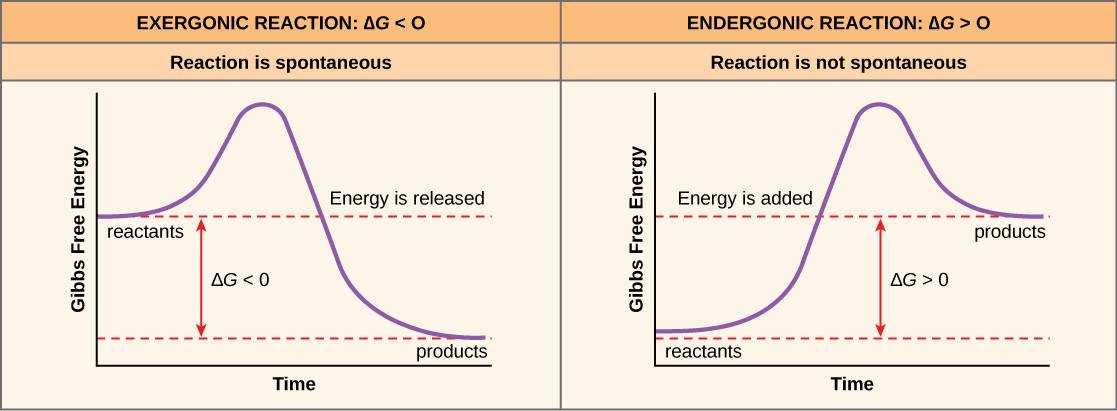
Where #DeltaG# is the free energy change (in #kJ mol^(-1)#) Using the equation #DeltaG = DeltaH -TDeltaS# Spontaneous Reaction and Gibbs Free Vitality 1-4 What Causes a Reaction to exist Spontaneous.


 0 kommentar(er)
0 kommentar(er)
