
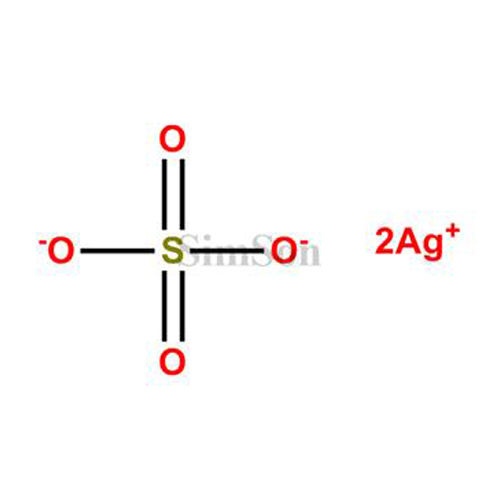
However, by using the solubility product (K sp) constant of Ag 2SO 4 and knowing the concentration at which the sulphate ions would precipitate out of the solution, we can determine the concentration of Ag 2+ ions. We cannot titrate HNO 3 + Ag 2SO 4 as Ag 2SO 4 is insoluble in aqueous solution. Ag 2SO 4 + 2HNO 3 -> 2 AgNO 3 + H 2SO 4 HNO 3 + Ag 2SO 4 titration.Thus, the overall balanced chemical reaction is as follows –.Add 2 moles of HNO 3 in the reactant side and 2 moles of AgNO 3 in the product side to balance the number of H, N and Ag atoms.We find that the number of moles of hydrogen, nitrogen and silver atoms in the reactant and product side are unequal.Elements Reactant side Product side H 1 2 N 1 1 O 7 7 S 1 1 Ag 2 1 Count of the elements in the reaction Determine the numbers of each element involved in the reaction, in both the reactant and product side.Given below are the steps to balance the above equation – The general chemical reaction of H NO 3 + Ag 2SO 4 can be represented as – HNO 3 + Ag 2SO 4 is a type of double decomposition reaction and acid formation reaction, as the cations and anions of the reactants exchange places to form silver nitrate salt and sulphuric acid.
Silver nitrate (AgNO 3) and sulphuric acid (H 2SO 4) are formed when Ag 2SO 4 reacts with concentrated HNO 3 .ĢHNO 3 + Ag 2SO 4 -> 2 AgNO 3 + H 2SO 4 What type of reaction is HNO 3 + Ag 2SO 4? What is the product of HNO 3 and Ag 2SO 4? In this section, let us focus on HNO 3 + Ag 2SO 4 reaction facts like the products formed, intermolecular forces involved, type of reaction, reaction enthalpy etc. It is a common reagent in laboratories and used in manufacturing fertilizers. Fresh HNO 3 is colorless and a powerful oxidizing agent. It is a neutral salt with weak oxidizing powers. Let us know more about their reaction mechanism.Īg 2SO 4 is a white inorganic solid with low solubility in water. Silver sulphate (Ag 2SO 4) is an inorganic silver salt, while Nitric acid (HNO 3) is an inorganic mineral oxoacid of nitrogen.


 0 kommentar(er)
0 kommentar(er)
